![]()
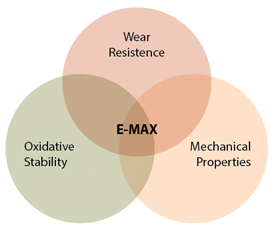
E-MAX Highly Crosslinked Polyethylene was developed to build on the lessons learned from the first generation of highly crosslinked polyethylenes (XLPE) and to address the trade-offs currently associated with these materials. In general, the goal was a material that fulfills three primary design requirements for a hip bearing: wear resistance, oxidative stability, and maintenance of mechanical properties.
Optimized crosslinking for wear resistance
First generation XLPE hip inserts have demonstrated very low wear rates in both hip simulators and in clinical radiographic studies. [1-9] Researches have found that the optimal radiation dose for improving wear resistance is about 10 Mrad, above which no further beneficial effects are observed.[10] Thus, engineers optimized the radiation dose for E-MAX, ensuring that the crosslink density for hip liners is comparable to 10 Mrad XLPE. [11]
Mechanically-annealed, rather than melt-annealed
Currently available XLPE is melt-annealed is to eliminate free radicals that lead to oxidation and subsequent polymer degradation. The reduction in mechanical properties caused by melt-annealing is well-known. [12-14] E-MAX is annealed using a proprietary mechanical compression process. This mechanical annealing eliminates free radicals—like melt-annealing—but does not diminish the mechanical properties of the polyethylene. [15]
Vitamin E-blended to address oxidation
Furthermore, recent retrieval studies of melt-annealed inserts have revealed unexpected signs of in vivo oxidation.[16,17] E-MAX contains 0.1%-weight vitamin E, which acts to scavenge free radicals, thus hindering the polyethylene oxidation cascade. [18,19]
Thus, E-MAX brings together the most important requirements for a polyethylene bearing: wear resistance, oxidative stability, and mechanical properties.
Learn more about E-MAX Highly Crosslinked Polyethylene.
- Kurtz S, Medel FJ, MacDonald D, Rimnac CM. In vivo oxidation, oxidation potential, and clinical performance of highly crosslinked UHMWPEs implanted for up to 8 years. 4th International Meeting UHMWPE for arthroplasty: From Powder to Debris 2009; Torino, Italy.
- Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: Two randomized studies using radiostereometric analysis. Acta Orthop 2007; 78(6): 746-54.
- D’Antonio JA, Manley MT, Capello WN, et al. Five-year experience with Crossfire highly cross-linked polyethylene. Clin Orthop Relat Res 2005; 441: 143-50.
- Engh CA, Stepniewski AS, Ginn SD, et al. A randomized prospective evaluation of outcomes after total hip Arthroplasty using crosslinked marathon and non-cross-linked Enduron polyethylene liners. J Arthroplasty 2006; 21(6): 17-25.
- Olyslaegers C, Defoort K, Simon JP, Vandenberghe L. Wear in conventional and highly cross-linked polyethylene cups: a 5-year follow-up study. J Arthroplasty 2008; 23(4): 489-94.
- Garcia-Rey E, Garcia-Cimbrelo E, Cruz-Pardos A, Ortega-Chamarro J. New polyethylenes in total hip replacement: a prospective, comparative clinical study of two types of liner. J Bone Joint Surg Br 2008; 90(2): 149-53.
- Geerdink CH, Grimm B, Vencken W, Heyligers IC, Tonino AJ. Cross-linked compared with historical polyethylene in THA: An 8-year clinical study. Clin Orthop Relat Res 2009; 467(4): 979-84.
- Glyn-Jones S, Isaac S, Hauptfleisch J, McLardy-Smith P, Murray DW, Gill HS. Does highly cross-linked polyethylene wear less than conventional polyethylene in total hip arthroplasty? A doubleblind, randomized, and controlled trial using roentgen stereophotogrammetric analysis. J Arthroplasty 2008; 23(3): 337-43.
- Kurtz SM, Medel FJ, MacDonald DW, Parvizi J, Kraay MJ, Rimnac CM. Reasons for revision of first-generation highly cross-linked polyethylenes. J Arthroplasty. 2010 Sep;25(6 Suppl):67-74.
- Moratoglu OK. Chapter 13 Highly crosslinked and melted UHMWPE. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.
- Materials Characterization Testing. Test Report TP0322. On file with KYOCERA Medical Technologies, Inc.
- Muratoglu, O. K., Wannomae, K. K., Rowell, S. L., Micheli, B. R., Malchau, H. Ex Vivo Stability Loss of Irradiated and Melted Ultra-High Molecular Weight Polyethylene. JBJS 2010;92:2809-2816.
- Oral E, Malhi AS, Muratoglu OK. Mechanisms of decrease in fatigue crack propagation resistance in irradiated and melted UHMWPE. Biomaterials 2006; 27(6):917-25.
- Rimnac C. On the mechanical properties of UHMWPE. Transactions of the 3rd UHMWPE International Meeting. Polyethylene in Total Joint Replacement Systems: Concerns and Solutions. Madrid, Spain. September 14-15, 2007.
- Bhattacharyya S, Matrisciano L, Spiegelberg S, Harris W, Muratoglu O. Mechanical elimination of residual free radicals in an irradiated UHMWPE rod: advantages over melting. 50th annual meeting of the orthopaedic research society. 2004:1474.
- Kurtz SM, MacDonald D, Brenner E, Medel FJ, Hozack W, Parvizi J, Goldberg V, Kraay M, Stulberg B, Rimnac CM. In vivo oxidation, oxidation potential, and clinical performance of first and second generation highly crosslinked acetabular bearings for THA. Poster No. 1790 54th Annual Meeting of the ORS, 2008.
- Currier BH, Van Citters D., Currier JH, Collier JP. In Vivo Oxidation in Remelted Highly Cross-Linked Retrievals. JBJS 2010; 92(14):2409-18.
- Kurtz S, Bracco, Costa L. Chapter 16 Vitamin-E-blended UHMWPE biomaterials. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.
- Costa L, Bracco P. Chapter 21 Mechanisms of crosslinking, oxidative degradation, and stabilization of UHMWPE. In UHMWPE Biomaterials Handbook Second Edition (ed. Kurtz SM). Elsevier: Amsterdam, 2009.