
Tesera® SA
Standalone ALIF Interbody Fusion System


Tesera Trabecular Technology®
- Optimal environment for bone on-growth and biologic fixation
- 3D-printed Titanium-alloy (Ti6AI4V)
- Truly-porous trabecular structure
- Random, interconnected pores ( >500 micron average pore size)
- 68% Average Porosity
- Hydroxyapetite-blasted, for micro-surface roughness
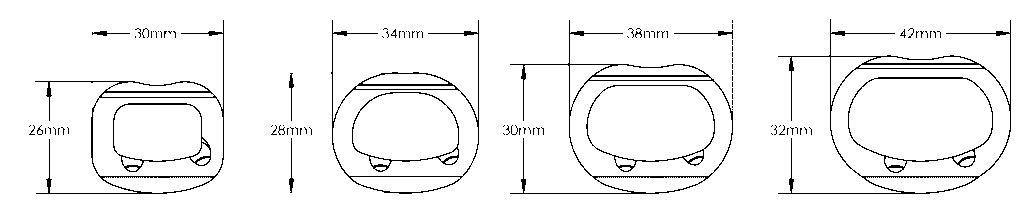
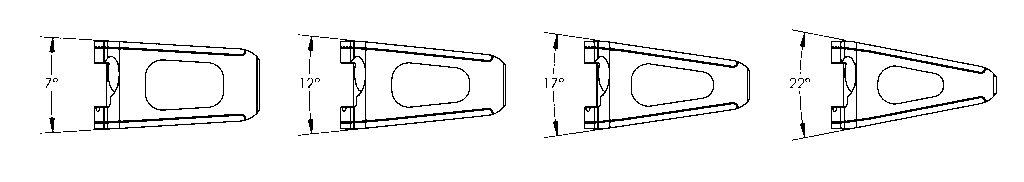
Size Array
- • 4 Lordotic Angles (7˚, 12˚, 17˚ and 22˚)
- Heights from 11mm – 19mm (2mm increments)
- 4.5mm and 5.0mm diameter Screws
- Screw lengths from 20mm – 35mm (5mm increments)
- 4 Footprints

Tesera SA 30mm x 26mm footprint shown
SA Lateral View
Simple, Secure Locking
- Single-step locking plate securely locks all four screws
- Tactile locking confirmation
- Visual locking confirmation
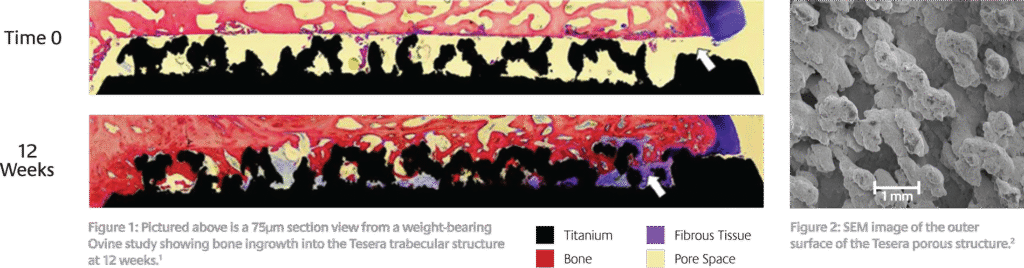
- References Surgeries were performed at IMDS Discovery Research (Logan, Utah); processing and analysis of the specimens was conducted by the Bone and Joint Research Laboratory (Salt Lake City, Utah). Data on file with Kyocera Medical Technologies, Inc.
- Data on file with Kyocera Medical Technologies, Inc. SEM Evaluation. Test Report K13047307-1.
- ** The Ovine study data shown is representative of Kyocera Medical Technologies’ Electron Beam additively manufactured porous structure. Tesera P/T/ST implants are manufactured using a laser sintering additively manufactured porous structure, but are representative of the Electron Beam porous structure.

KYOCERA Medical is committed to providing high quality orthopedic products that help surgeons meet the unique demands of every patient.
FIND US
HEADQUARTERS / WAREHOUSE
1289 Bryn Mawr Ave., Suite A.,
Redlands, CA 92374
R&D CENTER
10431 Morado Circle, Building V, Suite 150
Austin, TX 78759
©2025 Kyocera Medical Technologies, Inc. All rights reserved.