![]()
Tesera TLIF solutions feature our revolutionary Tesera Trabecular Technology in both a traditional curved, as well as straight formats, giving you maximum intraoperative options to fit any patient anatomy.
 |
|
| Tesera T and ST Transforaminal Lumbar Interbody Fusion (TLIF) System with Tesera Trabecular Technology |
|
KYOCERA is committed to advancing patient care through better products, based on scientific and clinical data. We commissioned an independent study through IMDS Discovery Research to validate our Tesera technology. Below are images and excerpts from the 12-Week Draft Report.
Tesera Trabecular Technology
- Optimal environment for bone IN-GROWTH and ON-GROWTH
- 3D-printed Titanium-alloy (Ti6Al4V)
- Truly-porous trabecular structure
- Random, interconnected pores (500 micron average pore size)
- 68% Average Porosit
- Hydroxyapetite-blasted, for micro-surface roughness

Tesera T (curved TLIF) Sizes
- Available in lengths of 28mm and 32mm X 10mm width
- Available in heights from 7mm – 16mm
- 5 ̊ lordotic profile
Tesera ST (straight TLIF) Sizes
- Available in lengths of 30mm and 34mm X 11mm width
- Available in heights from 7mm – 16mm
- Convex profile

Instruments
- Straight Shavers available from 6mm – 14mm (1mm increments)
- Curved and Straight Trials available from 7mm – 14mm (1mm increments)
- Threaded Inserters
- Tamps, disc prep and nerve retractors included
About Tesera Trabecular Technology (T3)…
Tesera implants feature porous titanium surfaces which create the optimal environment for bone on-growth and in-growth. (Figure 1) Independent study of the Tesera structure proves rapid and complete bone ingrowth at 12 weeks, without press-fit or biologics. (Figure 2)
Tesera implants combine revolutionary manufacturing technology, advanced material science and bio-analogous design into cutting-edge implants that push the expectations of how spinal implants interact with the body.

Figure 1: SEM image of the outer surface of the Tesera porous structure.[1]
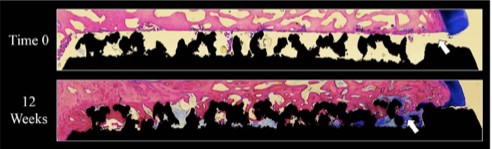
Figure 2: Pictured above is a 75μm section view from a weight-bearing Ovine study showing bone ingrowth into the Tesera trabecular structure at 12 weeks.[2] Black=Titanium, Pink=Bone, Blue=Fibrous Tissue and White=Pore Space
- Data on file with KYOCERA Medical Technologies, Inc. SEM Evaluation. Test Report K13047307-1.
- Surgeries were performed at IMDS Discovery Research (Logan, Utah); processing and analysis of the specimens was conducted by the Bone and Joint Research Laboratory (Salt Lake City, Utah). Data on file with KYOCERA Medical Technologies, Inc.
*The Ovine study data shown is representative of file with KYOCERA Medical Technologies’ Electron Beam additively manufactured porous structure. Tesera P/T/ST implants are manufactured using a laser sintering additively manufactured porous structure, but are representative of the Electron Beam porous structure.